Doping
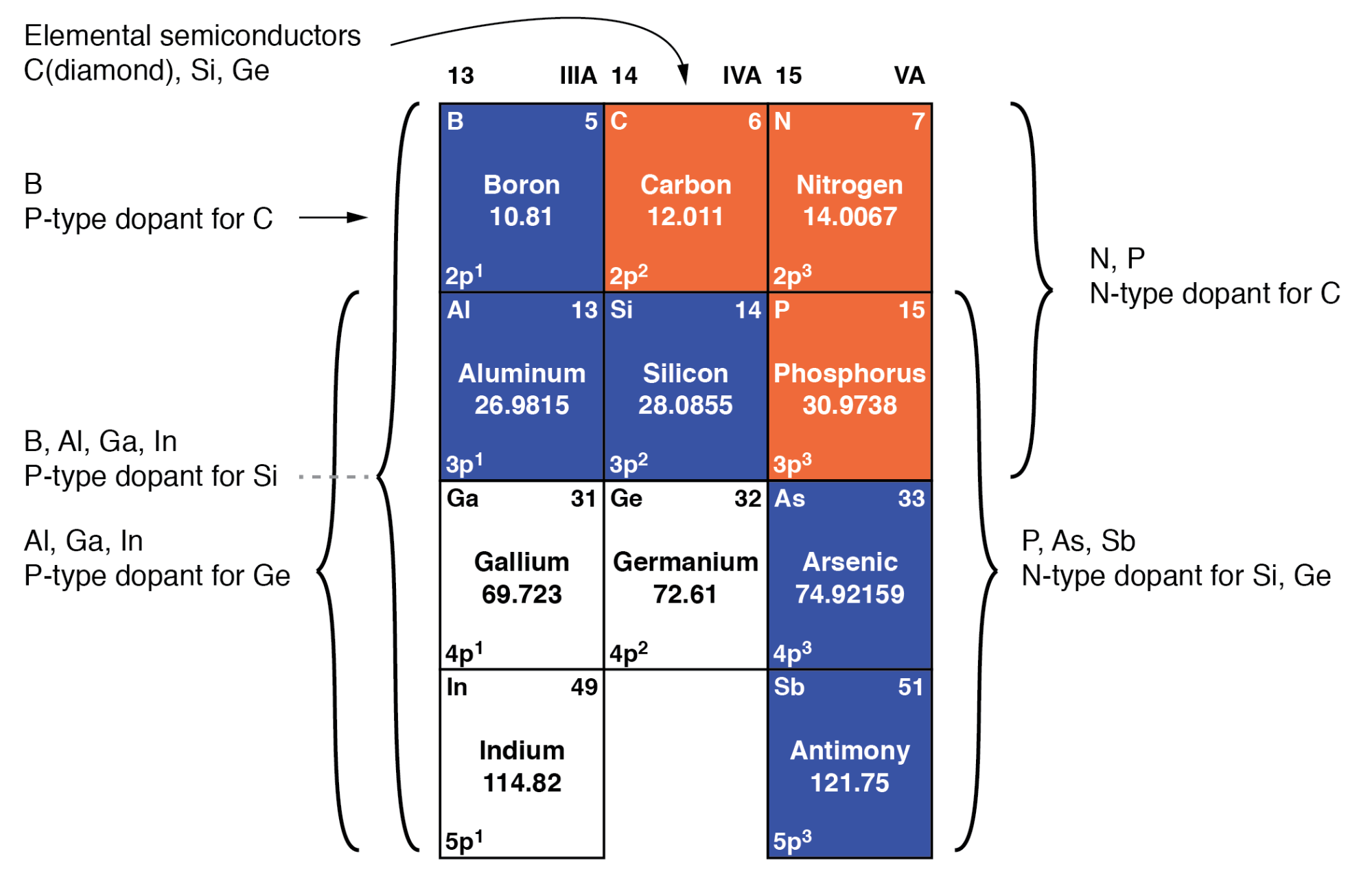
Doping is when we add impurities to the extremely pure silicon. This changes the electrochemical properties of the silicon. Silicon is in group 4 of the periodic table. Like Carbon it makes nice solid bonds in 4 directions. By adding elements from the 3rd or 5th columns we can add extra electrons or extra positive charges (holes where there are no electrons).
The elements we typically use are Boron for p-type (positive holes) doping and Phosphorus or Arsenic for n-type (extra electrons) doping. Undoped silicon has a very low conductivity meaning it does not conduct electricity, but it looks metallic. By adding small amounts of dopants it can be made to be conductive, and the voltage on transistor gates can change it from being insulating to conducting by controlling the way the extra (for N type) or missing (for P type) electrons behave.
The Arsenic is the poison in this fun tweet:
computers think using etchings in poisoned sand and measure time using vibrating crystals so if you were looking for magic you found it
— Computer Facts (@computerfact) April 20, 2016
For more information check this article from technocrazed
Course feedback
I've always wanted to make chips of my own since forever, but until this initiative it was unbelievably expensive. So I just needed to acquire the knowledge to do it. I saw you going through the whole thing from RTL to a design and that meant you had the insights for all of it.
Tommy Thorn (digital course)